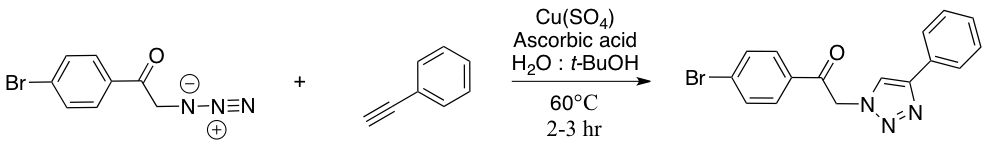
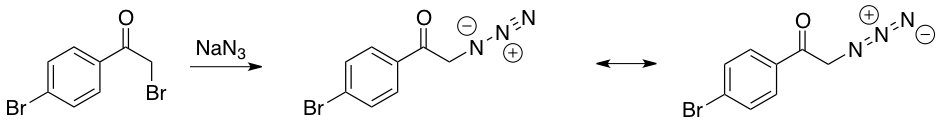
In this experiment, you will perform a one-pot reaction; that is, two distinct chemical transformations will be done in the same reaction vessel without isolation of the intermediate. The first reaction is a simple SN2 displacement of a bromide by an azide. Since low molecular weight organic azides are potentially explosive when isolated, this “one pot” procedure is one of the safest ways to handle organic azides. Afterwards, the generated azide will react with a terminal alkyne to yield a triazole; this cycloaddition reaction is relatively slow. The reaction is called a 1,3-dipolar cycloaddition, because a dipole (here a net positive and negative charge delocalized on the three nitrogen atoms of the azide) reacts with a dipolarphile (the alkyne) in a concerted manner (both ends of the dipole react at the same time) in a process similar to the Diels-Alder reaction, which is also a cycloaddition.
In the copper-catalyzed reaction, the role of the copper is to activate the terminal alkyne. However, this activation is normally done by a copper(I) species; you are adding copper sulfate, which has an oxidation state of +2. The role of the ascorbic acid is to reduce the oxidation state of the copper in situ from +2 to +1. While it is also possible to add Cu(I) directly to the reaction, these species are very expensive and tend to oxidize rapidly when stored on the shelf. For the proposed mechanism of the catalytic cycle, refer to reference 2.
The concept of Click Chemistry was introduced in by the Nobel Prize in Chemistry laureate K. Barry Sharpless.1 The basic principles of this approach are quite attractive in chemical synthesis for a number of reasons; in general, "click" reactions...
One of the most useful examples of Click Chemistry was proposed by Sharpless himself: the copper-catalyzed cycloaddition reaction between azides and terminal alkynes to generate triazoles.2 Triazoles are very interesting pharmacophores, and the modular aspect of this reaction permits the synthesis of triazole-based combinatorial libraries, which can be particularly useful in developing libraries of molecules for the study of structure-activity relationships in drug design. This reaction is also very reliable and does not lead to side reactions in the presence of other functional groups. Since it can be done in water, this reaction has been used in a wide range of biological applications, including the specific chemical modification of cell surface receptors3 and the conjugation of proteins to solid support for the fabrication of microarrays.4
To a 5 dram vial charged with 4-bromophenacyl bromide (556 mg) and a mini stir bar, tert-butanol (3 mL), water (3 mL), phenylacetylene (220 μL), and sodium azide (137 mg) were added. To form the catalyst, sodium ascorbate (40.0 mg) and 1 M aqueous copper (II) sulfate pentahydrate solution (100 μL) were added to the vial sequentially. The vial was capped and sealed with parafilm to ensure the stopper would stay in place. The vial was then heated for 1 hour at 60°C, with the stir bar moving rapidly enough to ensure good mixing of the two solvent layers. The reaction was monitored by TLC (thin layer chromatography) for disappearance of starting materials. (NOTE: If the starting material has disappeared by TLC, the reaction is complete; otherwise, continue the reaction for another 15 minutes and check the TLC again.) The reaction mixture was quenched by pouring it into 10-20 mL of ice-cold water; 10% aqueous ammonia solution (5mL) were added to the mixture in order to draw all the copper into the aqueous solution. The resulting mixture was stirred for 5 minutes, then filtered using a Buchner funnel to collect the solid precipitate. The precipitate was dried under vacuum, weighed and analyzed by MP, IR, and 1H NMR.
Prepare your notebook as described in the Notebook handout in myCourses.
Write a complete lab report according to the Lab Report Guidelines document on myCourses. Complete reports consist of all sections outlined in the Lab Report Guidelines: Abstract, Introduction, Results/Discussion, Experimental, and References